Details of the Drug Combinations
General Information of This Drug (ID: DMAKIDV)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dehydrogesterone; Didrogesterona; Didrogesterone; Diphaston; Dufaston; Duphaston; Duvaron; Dydrogesterona; Dydrogesteronum; Gestatron; Gynorest; Hydrogesterone; Hydrogestrone; Isopregnenone; Prodel; Retrone; Terolut; Didrogesterone [DCIT]; Solvay Brand of Dydrogesterone; DELTA6-Retroprogesterone; Duphaston (TN); Dydrogesterona [INN-Spanish]; Dydrogesteronum [INN-Latin]; Gynorest (TN); Delta(6)-Retroprogesterone; Delta(sup 6)-Retroprogesterone; Retro-6-dehydroprogesterone; Dydrogesterone (JP15/USP/INN); Dydrogesterone [USAN:INN:BAN:JAN]; Pregna-4,6-diene-3,20-dione, (9-beta,10-alpha)-(9CI); (8S,9R,10S,13S,14S,17S)-17-acetyl-10,13-dimethyl-1,2,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-one; (9-beta,10-alpha)-Pregna-4,6-diene-3,20-dione; (9beta,10alpha)-pregna-4,6-diene-3,20-dione; 10alpha-Isopregnenone; 6 Dehydro 9 beta 10 alpha progesterone; 6-Dehydro-9 beta-10 alpha-progesterone; 6-Dehydro-9.beta.,10.alpha.-progesterone; 6-Dehydro-9beta,10alpha-progesterone; 6-Dehydro-retro-progesterone; 6-Dehydroretroprogesterone; 9-.beta.,10.alpha.-Pregna-4,6-diene-3,20-dione; 9-beta,10-alpha-Pregna-4,6-diene-3,20-dione; 9-beta,10alpha-Pregna-4,6-diene-3,20-dione; 9.beta.,10.alpha.-Pregna-4,6-diene-3,20-dione; 9beta,10alpha-Pregna-4,6-diene-3,20-dione; 9beta,10alpha-Pregna-4,6-diene-3,20-dione (8CI)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Progesterones
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
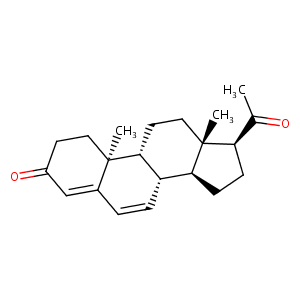 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
