Details of the Drug Combinations
General Information of This Drug (ID: DMB920Z)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ceritinib; LDK378; 1032900-25-6; ZYKADIA; NVP-LDK378-NX; LDK-378; UNII-K418KG2GET; LDK378(Ceritinib); LDK 378; Eritinib (LDK378); 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine; K418KG2GET; CHEMBL2403108; CHEBI:78432; AK174337; ceritinib; C28H36ClN5O3S; 5-Chloro-N2-[2-isopropoxy-5-Methyl-4-(4-piperidyl)phenyl]-N4-(2-isopropylsulfonylphenyl)pyriMidine-2,4-diaMine; 5-Chloro-N2-(5-methyl-4-(piperidin-4-yl)-2-(propan-2-yloxy)phenyl)-N4-(2-(propane-2-sulfonyl)phenyl)pyrim
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
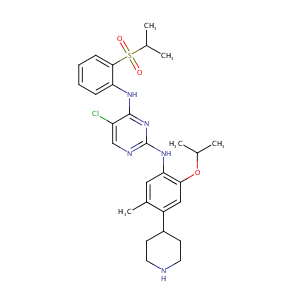 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
25 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
