Details of the Drug Combinations
General Information of This Drug (ID: DMBFE74)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ixel; Midalcipran; Milnacipranum; Toledomin; Milnacipran [INN]; Milnacipranum [Latin]; F 2207; F-2207; Ixel (TN); Milnacipran(INN); Savella (TN); (+-)-cis-2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide; (1R,2S)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropane-1-carboxamide; 1-phenyl-1-diethylaminocarbonyl-2-aminomethylcyclopropane HCl
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antidepressants
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
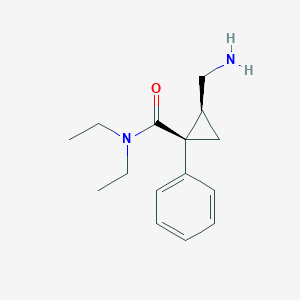 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
