Details of the Drug Combinations
General Information of This Drug (ID: DMBR8NF)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
VWMJHAFYPMOMGF-ZCFIWIBFSA-N; 1096708-71-2; MLN 2480; BIIB-024; Tak-580; MLN-2480; BIIB024; UNII-ZN90E4027M; BIIB 024; 4-Pyrimidinecarboxamide, 6-amino-5-chloro-N-[(1R)-1-[5-[[[5-chloro-4-(trifluoromethyl)-2-pyridinyl]amino]carbonyl]-2-thiazolyl]ethyl]-; ZN90E4027M; (r)-2-(1-(6-amino-5-chloropyrimidine-4-carboxamido)ethyl)-n-(5-chloro-4-(trifluoromethyl)pyridin-2-yl)thiazole-5-carboxamide; 4-Pyrimidinecarboxamide, 6-amino-5-chloro-N-((1R)-1-(5-(((5-chloro-4-(trifluoromethyl)-2-pyridinyl)amino)carbonyl)-2-thiazolyl)ethyl]-; AMG 2112819
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
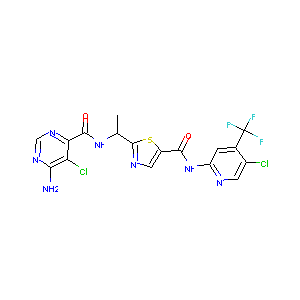 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
