Details of the Drug Combinations
General Information of This Drug (ID: DMC7XDN)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alcelam; Algad; Alpaz; Alplax; Alpram; Alprax; Alprazolamum; Alprazolan; Alpronax; Alprox; Alviz; Alzam; Alzolam; Alzon;Anpress; ApoAlpraz; Azor; Bestrol; Cassadan; Constan; Esparon; Frontal; Helex; Intensol; Ksalol; Mialin; Neurol; Niravam; NovoAlprazol; NuAlpraz; Panistat; Panix; Pharnax; Prazam; Prazolan; Prinox; Ralozam; Relaxol; Restyl; Solanax; Tafil; Tensivan; Tranax; Trankimazin; Tranquinal; Tricalma; Unilan; Valeans; Xanagis; Xanax; Xanolam; Xanor; Zacetin; Zanapam; Zaxan; Zenax; Zolam; Zolan; Zolarem; Zoldac; Zoldax; Zopax; Zopic; Zotran; Alphapharm Brand of Alprazolam; Alprazolam Alphapharm Brand; Alprazolam Apotex Brand; Alprazolam Kenral Brand; Alprazolam Novopharm Brand; Alprazolam Orion Brand; Alprazolam Pfizer Brand; Alprazolam Temmler Brand; Alprazolam extended release tablets; Alprazolam intensol; Alprazolam solution; Apo Alpraz; Apotex Brand of Alprazolam; Arzneimittelwerk Dresden Brand of Alprazolam; Kenral Brand of Alprazolam; Novo Alprazol; Novopharm Brand of Alprazolam; Nu Alpraz; Nu Pharm Brand of Alprazolam; Orion Brand of Alprazolam; Pfizer Brand of Alprazolam; Tafil D; Temmler Brand of Alprazolam; Xanax TS; Xanax XR; D 65MT; D65MT; TGAR01P; Tus 1; U 31889; AP-1002; AZ-002; Alprazolam Nu-Pharm Brand; Alprazolam-d5; Alprazolamum [INN-Latin]; Apo-Alpraz; D-65MT; Gen-Alprazolan; Niravam (TN); Novo-Alprazol; Nu-Alpraz; Nu-Pharm Brand of Alprazolam; Staccato-alprazolam; TUS-1; U-31889; U31,889; Xanax (TN); Xanor (TN); U-31,889; Alprazolam (JP15/USP/INN); Alprazolam [USAN:INN:BAN:JAN]; 8-Chloro-1-methyl-6-(phenyl-d5)-4H-(1,2,4)triazolo[4,3-a][1,4]benzodiazepine; 8-Chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine; 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo(4,3-a)(1,4)benzodiazepine; 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo(4,3-alpha)(1,4)benzodiazepine; 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
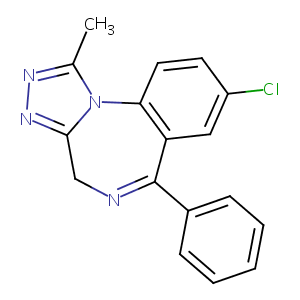 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
