Details of the Drug Combinations
General Information of This Drug (ID: DMCVXBE)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
E64D; E64d; Aloxistatin; Aloxistatin [INN]; Aloxistatina; Aloxistatina [Spanish]; Aloxistatine; Aloxistatine [French]; Aloxistatinum; Aloxistatinum [Latin]; BRN 5354546; C17H30N2O5; CCRIS 1934; CHEMBL63440; E 64c ethyl ester; E 64d; EP 453; EST; EST (pharmaceutical); L5W337AOUR; Loxistatin; MLS000028372; NSC 694281; SMR000058552; UNII-L5W337AOUR
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
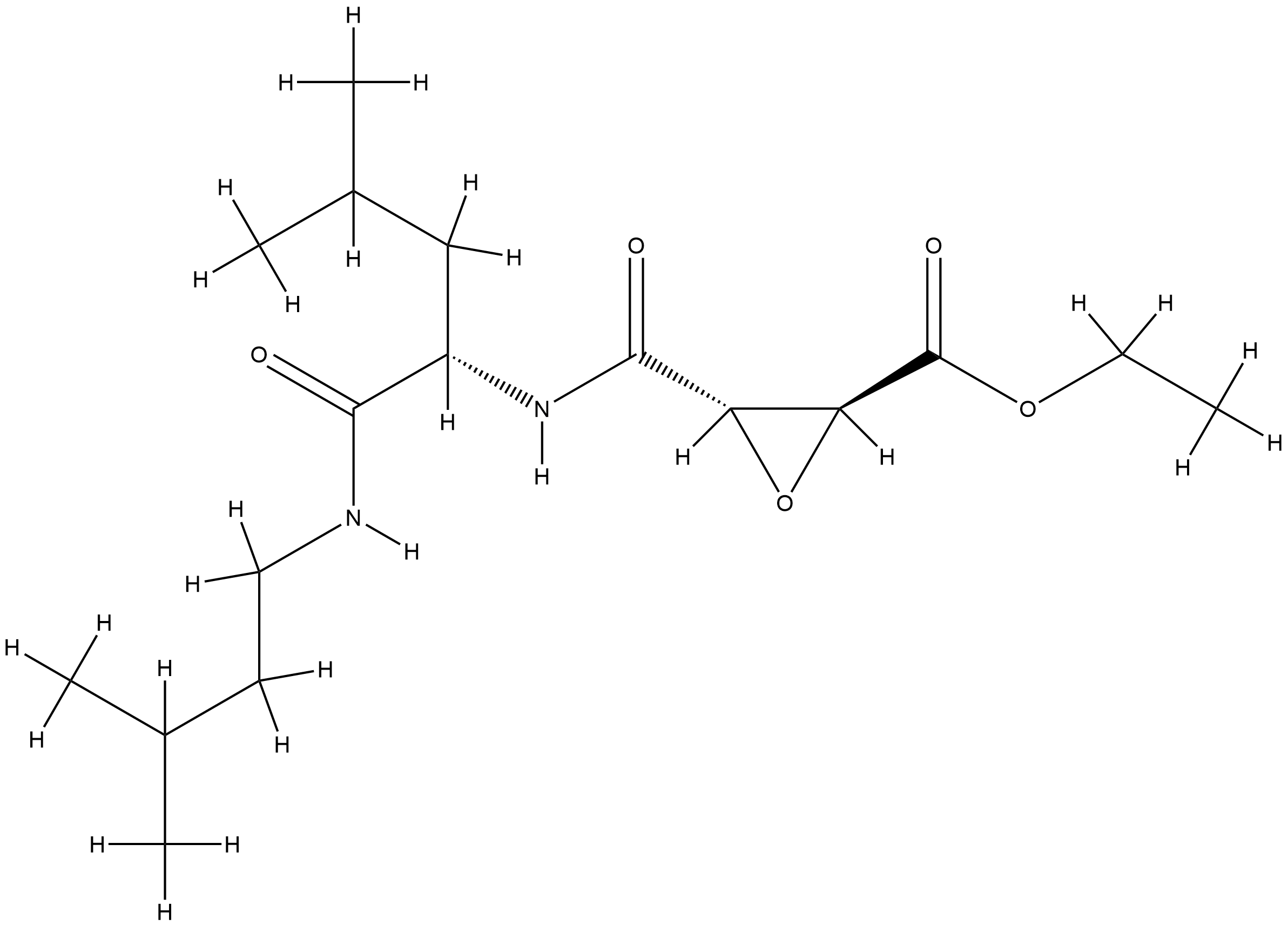 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
