Details of the Drug Combinations
General Information of This Drug (ID: DMCZH5B)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GS-9256; UNII-8V42Y78HRU; 1001094-46-7; GS 9256; 8V42Y78HRU; [[8-chloro-2-[2-(isopropylamino)thiazol-4-yl]-7-methoxy-4-quinolyl]oxy-(cyclopentoxycarbonylamino)-dioxo-[ ]yl]-[(2,6-difluorophenyl)methyl]phosphinic acid; GS9256; SCHEMBL9987287; CHEMBL1956820; DTXSID70142946; DB12876
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
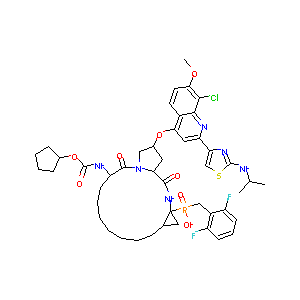 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
