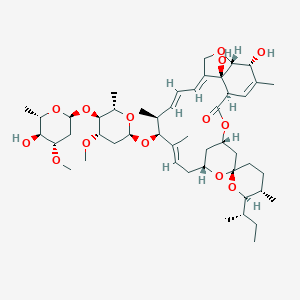
| 1 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2373).
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 202736
|
| 3 |
Ivermectin FDA Label
|
| 4 |
ClinicalTrials.gov (NCT04351347) The Efficacy of Ivermectin and Nitazoxanide in COVID-19 Treatment. U.S. National Institutes of Health.
|
| 5 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 6 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 7 |
ClinicalTrials.gov (NCT02974049) Lymphatic Filariasis (LF) in Ivory Coast
|
| 8 |
ClinicalTrials.gov (NCT02046200) Development of Ivermectin for Alcohol Use Disorders
|
| 9 |
ClinicalTrials.gov (NCT04714515) Montelukast - a Treatment Choice for COVID-19
|
| 10 |
ClinicalTrials.gov (NCT03664063) PK PD Study of IDA and Azithromycin for NTDs ( ComboNTDs )
|
| 11 |
ClinicalTrials.gov (NCT04712279) The (HD)IVACOV Trial (The High-Dose IVermectin Against COVID-19 Trial)
|
| 12 |
ClinicalTrials.gov (NCT02509481) Repeat Ivermectin Mass Drug Administrations for Control of Malaria: a Pilot Safety and Efficacy Study
|
| 13 |
ClinicalTrials.gov (NCT04447235) Early Treatment With Ivermectin and LosarTAN for Cancer Patients With COVID-19 Infection
|
| 14 |
ClinicalTrials.gov (NCT04410406) Moxidectin for LF, Cote d'Ivoire (DOLF)
|
| 15 |
ClinicalTrials.gov (NCT03236168) Impact of Community Scabies Treatment on Head Lice Prevalence in the Solomon Islands
|
| 16 |
ClinicalTrials.gov (NCT04374019) Novel Agents for Treatment of High-risk COVID-19 Positive Patients. U.S. National Institutes of Health.
|
| 17 |
ClinicalTrials.gov (NCT04510194) COVID-OUT: Early Outpatient Treatment for SARS-CoV-2 Infection (COVID-19)
|
| 18 |
ClinicalTrials.gov (NCT04382846) Novel Regimens in COVID-19 Treatment. U.S. National Institutes of Health.
|
| 19 |
ClinicalTrials.gov (NCT04360356) Ivermectin and Nitazoxanide Combination Therapy for COVID-19. U.S. National Institutes of Health.
|
| 20 |
ClinicalTrials.gov (NCT04729140) An Outpatient Clinical Trial Using Ivermectin and Doxycycline in COVID-19 Positive Patients at High Risk to Prevent COVID-19 Related Hospitalization
|
| 21 |
ClinicalTrials.gov (NCT02775617) Azithromycin - Ivermectin Mass Drug Administration for Skin Disease
|
| 22 |
ClinicalTrials.gov (NCT02616250) MirvasO Soolantra Association In the Treatment of Moderate to Severe rosaCea.
|
|
|
|
|
|
|