Details of the Drug Combinations
General Information of This Drug (ID: DMDF79Z)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
acetylcholine; Choline acetate; O-Acetylcholine; Acetyl choline ion; Acetylcholinum; 51-84-3; (2-Acetoxyethyl)trimethylammonium; Acetyl choline cation; Choline acetate (ester); 2-(Acetyloxy)-N,N,N-trimethylethanaminium; Ach; Azetylcholin; ethanaminium, 2-(acetyloxy)-N,N,N-trimethyl-; UNII-N9YNS0M02X; BRN 1764436; CHEBI:15355; Bromoacetylcholine; EINECS 200-128-9; CHEMBL667; N9YNS0M02X; [2-(acetyloxy)ethyl]trimethylazanium; Ethanaminium, 2-(acetyloxy)-N,N,N-trimethyl- (9CI); 2-acetyloxyethyl-trimethylazanium; [3H]acetylcholine; Miochol; Miochol-e
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Cardiovascular Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
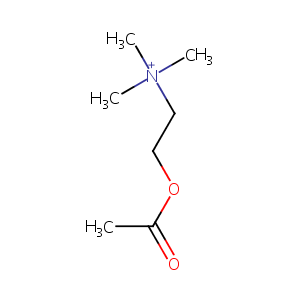 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
