Details of the Drug Combinations
General Information of This Drug (ID: DMDG8Q3)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
VARLITINIB; 845272-21-1; ARRY-334543; ARRY334543; ARRY 334543; ARRY-543; UNII-846Y8197W1; ASLAN-001; 846Y8197W1; (R)-N4-(3-chloro-4-(thiazol-2-ylmethoxy)phenyl)-N6-(4-methyl-4,5-dihydrooxazol-2-yl)quinazoline-4,6-diamine; Varlitinib [USAN:INN]; Varlitinib (ARRY334543); Varlitinib free base; Varlitinib (USAN/INN); MLS006011274; GTPL7645; SCHEMBL1384578; CHEMBL2103842; SYN1192; MolPort-028-720-424; EX-A1005; 4-N-[3-chloro-4-(1,3-thiazol-2-ylmethoxy)phenyl]-6-N-[(4R)-4-methyl-4,5-dihydro-1,3-oxazol-2-yl]quinazoline-4,6-diamine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
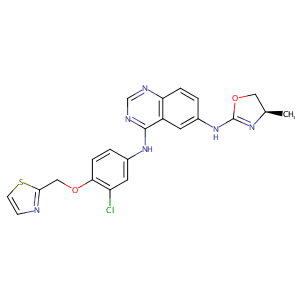 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
