Details of the Drug Combinations
General Information of This Drug (ID: DME47TX)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
RO5045337; QBGKPEROWUKSBK-QPPIDDCLSA-N; 939981-39-2; UNII-Q8MI0X869M; Q8MI0X869M; RO-5045337; CHEMBL2386346; Ro 5045337; GTPL9599; SCHEMBL12704861; DTXSID60240182; MolPort-044-560-282; EX-A1686; s7030; ZINC96270381; BDBM50434287; AKOS027282701; CS-0330; HY-10959; RG7112 (RO5045337); [(4s,5r)-2-(4-t-butyl-2-ethoxyphenyl)-4,5-bis(4-chlorophenyl)-4,5-dimethylimidazol-1-yl]-[4-(3-methylsulfonylpropyl)piperazin-1-yl]methanone; Methanone, [(4S,5R)-4,5-bis(4-chlorophenyl)-2-[4-(1,1-dimethylethyl)-2-ethoxyphenyl]
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
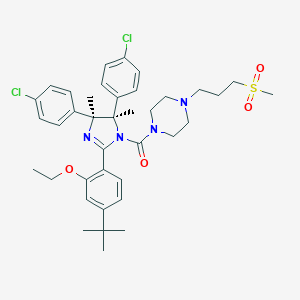 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
