Details of the Drug Combinations
General Information of This Drug (ID: DME53YS)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Sotrastaurin acetate; UNII-R1SIA15KZ1; 908351-31-5; R1SIA15KZ1; Sotrastaurin acetate [USAN]; AEB 071; Sotrastaurin acetate (USAN); SCHEMBL3846239; CHEMBL2105655; KB-74525; D09718; 3-(1H-Indol-3-yl)-4-(2-(4-methylpiperazin-1-yl)quinazolin-4-yl)-1H-pyrrole-2,5-dione acetate; 1H-Pyrrole-2,5-dione, 3-(1H-indol-3-yl)-4-(2-(4-methyl-1-piperazinyl)-4-quinazolinyl)-, acetate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
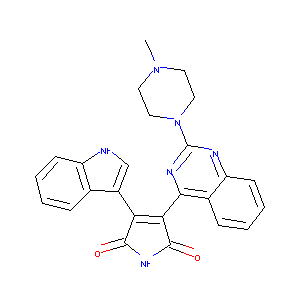 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
