Details of the Drug Combinations
General Information of This Drug (ID: DME78OA)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Akineton; Beperiden; Biperidene; Biperideno; Biperidenum; Biperidine; Biperidene hydrochloride; KL 373; Akineton (TN);Biperidene [INN-French]; Biperideno [INN-Spanish]; Biperidenum [INN-Latin]; Biperiden (JAN/USP/INN); Biperiden [USAN:BAN:INN:JAN]; Biperiden [USAN:INN:BAN:JAN]; Alpha-5-Norbornen-2-yl-alpha-phenyl-1-piperidinepropanol; Alpha-bicyclo[2.2.1]hept-5-en-2-yl-alpha-phenyl-1-piperidinepropanol; Alpha-Bicyclo(2.2.1)hept-5-en-2-yl-alpha-phenyl-1-piperidinepropanol; Alpha-(Bicyclo(2.2.1)hept-5-en-2-yl)-alpha-phenyl-1-piperidino propanol; 1-(5-bicyclo[2.2.1]hept-2-enyl)-1-phenyl-3-piperidin-1-ylpropan-1-ol; 1-(Bicyclo(2.2.1)hept-5-en-2alpha-yl)-1-phenyl-3-piperidinopropanol; 1-(bicyclo[2.2.1]hept-5-en-2-yl)-1-phenyl-3-(piperidin-1-yl)propan-1-ol; 1-Bicycloheptenyl-1-phenyl-3-piperidino-propanol-1; 1-Piperidinepropanol, .alpha.-bicyclo[2.2.1]hept-5-en-2-yl-.alpha.-phenyl-, hydrochloride; 3-Piperidino-1-phenyl-1-bicyclo(2.2.1)hepten-(5)-yl-propanol-(1); 3-Piperidino-1-phenyl-1-bicyclo(2.2.1)hepten-(5)-yl-propanol-(1) [German]; 3-Piperidino-1-phenyl-1-bicycloheptenyl-1-propanol
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiparkinson Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
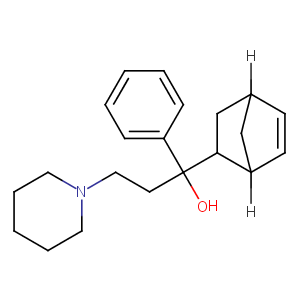 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
14 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
