Details of the Drug Combinations
General Information of This Drug (ID: DMEB837)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cefotaxima; Cefotaxima [INN-Spanish]; Cefotaxime [INN:BAN]; Cefotaxime acid; Cefotaximum; Cefotaximum [INN-Latin]; Cephotaxime; E-cefotaxime; Omnatax; RU 24662; RU 24756; cefotaxime; 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 3-((acetyloxy)methyl)-7-(((2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-8-oxo-, (6R,7R)-; 60846-21-1; 63527-52-6; C16H17N5O7S2; CHEBI:204928; EINECS 264-299-1; HR 756; N2GI8B1GK7; Taxim; UNII-N2GI8B1GK7
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
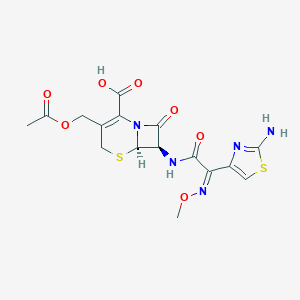 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
