Details of the Drug Combinations
General Information of This Drug (ID: DMEBOFW)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
clofazimine; 2030-63-9; Lamprene; Lampren; Chlofazimine; Clofazimina; Clofaziminum; Clofaziminum [INN-Latin]; B 663 (Pharmaceutical); Clofazimina [INN-Spanish]; B-663; NSC-141046; G 30320; UNII-D959AE5USF; B 663 (VAN); C27H22Cl2N4; 3-(p-Chloranilino)-10-(p-chlorphenyl)-2,10-dihydro-2-(isopropylimino)-phenazin; B 663; 3-(p-Chloroanilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)phenazine; 3-(p-Chloranilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)-phenazine; EINECS 217-980-2; CFZ; SMP2_000339; B 663, pharmaceutical; G-30320; Lamprene (TN); Liposome-encapsulated clofazimine; Clofazimine [USAN:INN:BAN]; G-30,320; Clofazimine (JAN/USP/INN); N,5-bis(4-chlorophenyl)-3-propan-2-yliminophenazin-2-amine; N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-(isopropylimino)phenazin-2-amine; N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-(1-methylethyl)imino)-2-phenazinamine; N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-[(1-methylethyl)imino]-2-phenazinamine; N,5-bis(4-chlorophenyl)-3-(propan-2-ylimino)-3,5-dihydrophenazin-2-amine; (3Z)-N,5-bis(4-chlorophenyl)-3-[(1-methylethyl)imino]-3,5-dihydrophenazin-2-amine; 3-(p-Chloranilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)phenazine; 3-(p-Chloranilino)-10-(p-chlorphenyl)-2,10-dihydro-2-(isopropylimino)-phenazin [German]; Riminophenazine
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
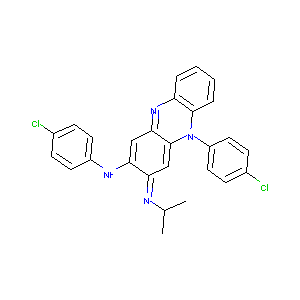 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
