Details of the Drug Combinations
General Information of This Drug (ID: DMEVAOB)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
183293-82-5; UNII-B96UX1DDKS; B96UX1DDKS; 6-(5-Carboxy-5-methyl-hexyloxy)-2,2-dimethyl-hexanoic acid; Gemcabene [INN]; PD-72953; PD 72953; AC1L4IHZ; SCHEMBL761631; CHEMBL2110686; CTK4D8457; DTXSID60171407; SDMBRCRVFFHJKR-UHFFFAOYSA-N; ZINC1893031; PD72953; DB05123; ACM183293825; 6,6'-Oxybis(2,2-dimethylhexanoic acid); Hexanoic acid,6,6'-oxybis[2,2-dimethyl-; FT-0743032; 2,2,2',2'-Tetramethyl-6,6'-oxydihexanoic acid; 6-(6-hydroxy-5,5-dimethyl-6-oxohexoxy)-2,2-dimethylhexanoic acid; 6-(5-CARBOXY-5-METHYL-HEXOXY)-2,2-DIME
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
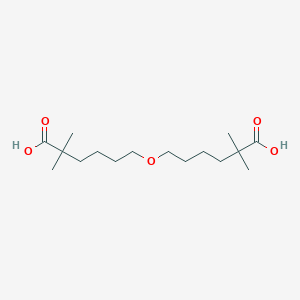 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
