Details of the Drug Combinations
General Information of This Drug (ID: DMFY0MX)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tedizolid phosphate; Torezolid phosphate; 856867-55-5; Tedizolid (phosphate); UNII-O7DRJ6R4DW; CHEBI:83326; O7DRJ6R4DW; TR-701; TR-701 FA; TR-701-FA; (R)-(3-(3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl dihydrogen phosphate; NE63553; [(5R)-3-{3-fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl]phenyl}-2-oxo-1,3-oxazolidin-5-yl]methyl dihydrogen phosphate; (R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one dihydrogenphos
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
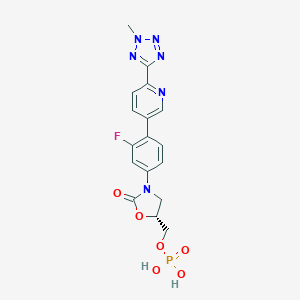 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
