Details of the Drug Combinations
General Information of This Drug (ID: DMHM7JS)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Signifor; UNII-I4P76SY3N4; Pasireotide diaspartate; I4P76SY3N4; SOM230; 820232-50-6; pasireotide aspartate; Pasireotide diaspartate [EMA EPAR]; CHEBI:72313; Cyclo((2R)-2-phenylglycyl-d-tryptophyl-l-lysyl-o-(phenylmethyl)-l-tyrosyl-l-phenylalanyl-(4R)-4-((((2-aminoethyl)amino)carbonyl)oxy)-l-prolyl), l-aspartate; Signifor (TN); SOM 230; SOM-230
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
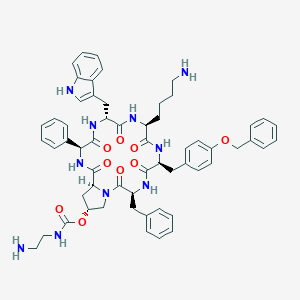 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
