Details of the Drug Combinations
General Information of This Drug (ID: DMHP21E)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
hydromorphone; Dihydromorphinone; Dimorphone; Idromorfone; Hydromorphon; Dihydromorfinon; Hydromorfona; Laudacon; DiMo; Hydromorphonum; 7,8-Dihydromorphinone; Novolaudon; Hidromorfona; Dilaudid; Dilaudid Oros; Hydromorfona [Spanish]; Dihydromorfinon [Czech]; 466-99-9; Hydromorphonum [INN-Latin]; Hidromorfona [INN-Spanish]; Palladone; 6-Deoxy-7,8-dihydro-6-oxomorphine; Morphinone, dihydro-; Idromorfone [DCIT]; Laudicon; Hydromorphone [INN:BAN]; Dilaudid-hp; 4,5-Epoxy-3-hydroxy-17-methylmorphinan-6-one; DiMo; Hydromorphone HCL; Dilaudid (TN); Hydromorphone (INN); Palladone (TN); Palladone SR (TN); Morphinan-6-one, 4,5alpha-epoxy-3-hydroxy-17-methyl-(8CI); (-)-(5R)-4,5-Epoxy-3-hydroxy-9alpha-methylmorphinan-6-one; (-)-Hydromorphone; (5alpha)-3-hydroxy-17-methyl-4,5-epoxymorphinan-6-one; 3-hydroxy-17-methyl-4,5alpha-epoxymorphinan-6-one; 4,5alpha-Epoxy-3-hydroxy-17-methyl-6-morphinanone; Hydromorphone prodrug
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
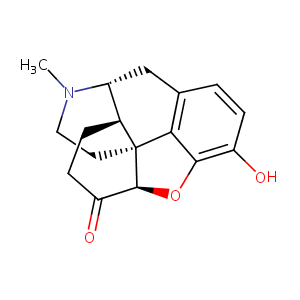 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
7 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
