Details of the Drug Combinations
General Information of This Drug (ID: DMHT34O)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
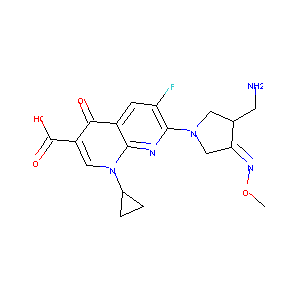
Factiv; Factive; Gemifloxacin [INN]; Gemifloxacin mesilate; LB 20304; LB 20304a; SB 265805; Factiv (TN); Factive (TN); Gemifloxacin (INN); LB-20304; SB-265805; 7-(3-Aminomethyl)-4-methoxyimino-pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid; 7-(3-Aminomethyl-4-methoxyimino-pyrrolidine-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-(1,8)-naphthyridine-3-carboxylic acid; 7-[3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
