Details of the Drug Combinations
General Information of This Drug (ID: DMHTG8P)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
YUAALFPUEOYPNX-UHFFFAOYSA-N; 1341200-45-0; TP0903; UNII-14D65TV20J; CHEMBL2022968; compound 13; 14D65TV20J; TP 0903; 2-((5-chloro-2-((4-((4-methylpiperazin-1-yl)methyl)phenyl)amino)pyrimidin-4-yl)amino)-N,N-dimethylbenzenesulfonamide; GTPL8863; SCHEMBL12813478; EX-A609; MolPort-039-193-844; ZINC84617535; BDBM50382425; AKOS026750306; CS-4281; DA-45909; HY-12963; S7846; FT-0700169; B5940; J-690134; 2-{[5-chloro-2-({4-[(4-methylpiperazin-1-yl)methyl]phenyl}amino)pyrimidin-4-yl]amino}-N,N-dimethylbenzene-1-sulfonamide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
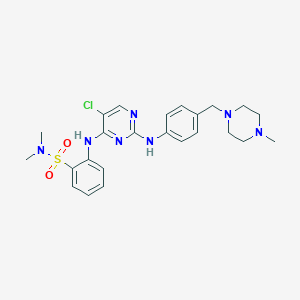 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
