Details of the Drug Combinations
General Information of This Drug (ID: DMI782S)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1346242-81-6; UNII-890E37NHMV; 890E37NHMV; Erdafitinib [USAN:INN]; Erdafitinib (USAN/INN); GTPL9039; SCHEMBL2583760; CHEMBL3545376; MolPort-044-560-398; JNJ-42756493 (Erdafitinib); s8401; compound 4 [WO2011135376]; ZINC168520308; AKOS030526429; CS-4988; DB12147; AC-30222; 1,2-Ethanediamine, N1-(3,5-dimethoxyphenyl)-N2-(1-methylethyl)-N1-(3-(1-methyl-1H-pyrazol-4-yl)-6-quinoxalinyl)-; HY-18708; AS-35040; KB-333716; D10927; N'-(3,5-dimethoxyphenyl)-N'-[3-(1-methylpyrazol-4-yl)quino
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Structure |
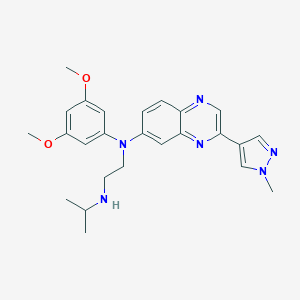 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
