Details of the Drug Combinations
General Information of This Drug (ID: DMIYHAW)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PU-H71; 873436-91-0; PU H71; PU-H 71; UNII-06IVK87M04; CHEMBL200102; 06IVK87M04; 6-amino-8-[(6-iodo-1,3-benzodioxol-5-yl)thio]-n-(1-methylethyl)-9h-purine-9-propanamine; NSC 750424; 8-[(6-Iodo-1,3-Benzodioxol-5-Yl)thio]-9-[3-(Isopropylamino)propyl]-9h-Purin-6-Amine; 8-(6-Iodobenzo[d][1,3]dioxol-5-ylthio)-9-(3-(isopropylamino)propyl)-9H-purin-6-amine; 8-((6-Iodobenzo[d][1,3]dioxol-5-yl)thio)-9-(3-(isopropylamino)propyl)-9H-purin-6-amine; H71; PUH-71; 2fwz; PU-H-71
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
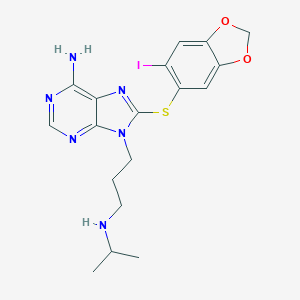 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
