Details of the Drug Combinations
General Information of This Drug (ID: DMJ7IKW)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PF-04494700; TTP-488; TTP-488); Alzheimers therapy, TransTech/Pfizer; Diabetic nephropathy therapy, Transtech/Pfizer; Alzheimer'streatment, TransTech/Pfizer; Alzheimer's therapy (RAGE), Transtech/Pfizer
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
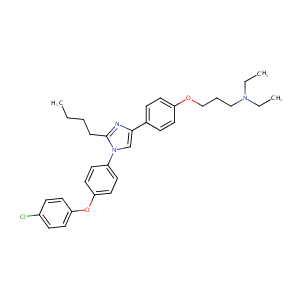 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
