Details of the Drug Combinations
General Information of This Drug (ID: DMJKHDL)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ioversol; 87771-40-2; Optiray; Optiray 320; Ioversolum [Latin]; Optiray 240; Optiray 350; Optiray 160; MP-328; UNII-N3RIB7X24K; LOVERSOL; BRN 7155654; N3RIB7X24K; MP 328; 1,3-Benzenedicarboxamide, N,N'-bis(2,3-dihydroxypropyl)-5-((hydroxyacetyl)(2-hydroxyethyl)amino)-2,4,6-triiodo-; DSSTox_RID_80927; DSSTox_CID_25521; N,N'-Bis(2,3-dihydroxypropyl)-5-(N-(2-hydroxyethyl)glycolamido)-2,4,6-triiodoisophthalamide; DSSTox_GSID_45521; Optiray 300; Ioversolum
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
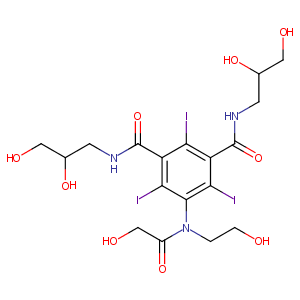 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
