Details of the Drug Combinations
General Information of This Drug (ID: DMJLE18)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Elbasvir; Elbasvir [USAN:INN]; Elbasvir(MK-8742); MK 8742; MK-8742; MK8742; SB16741; ZINC150588351; 1370468-36-2; 632L571YDK; A16855; CHEBI:132967; CHEMBL3039514; CS-5332; Carbamic acid, N,N'-(((6S)-6-phenyl-6H-indolo(1,2-c)(1,3)benzoxazine-3,10-diyl)bis(1H-imidazole-5,2-diyl-(2S)-2,1-pyrrolidinediyl((1S)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl)))bis-, C,C'-dimethyl ester; DB11574; EX-A2889; HY-15789; SCHEMBL17429773; UNII-632L571YDK
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
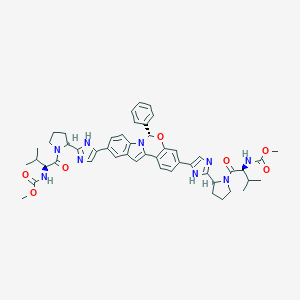 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
|
1 Approved Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
