Details of the Drug Combinations
General Information of This Drug (ID: DMKP45D)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Beprana; Nitronaproxen; AR-P900758XX; AZD-3582; HCT-3012; Nitronaproxen, NicOx; NO-naproxen, AstraZeneca; NO-naproxen, NicOx; NO-NSAID, NicOx/AstraZeneca; Nitric oxide-donating derivative of naproxen (oral, osteoarthritis), NicOx
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
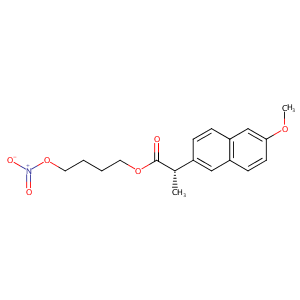 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
