Details of the Drug Combinations
General Information of This Drug (ID: DML634R)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Beraprost [USAN:INN]; Beraprostum; Beraprostum [INN-Latin]; MDL-201229; ML-1229; beraprost; (+-)-(1R,2R,3aS,8bS)-2,3,3a,8b-Tetrahydro-2-hydroxy-1-((E)-(3S,4RS)-3-hydroxy-4-methyl-1-octen-6-ynyl)-1H-cyclopenta(b)benzofuran-5-butyric acid; 2-Hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-ynyl)-2,3,3a,8b-tetrahydro-1H-cyclopenta(b)benzofuran-5-butanoic acid; 88430-50-6; AC1OCEUA; CHEBI:135633; CHEMBL1207745; CTPOHARTNNSRSR-APJZLKAGSA-N; DTXSID7049136; SCHEMBL16904620; SCHEMBL34593
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
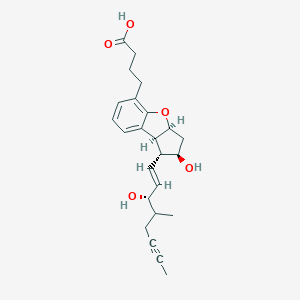 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
