| 1 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7367).
|
| 2 |
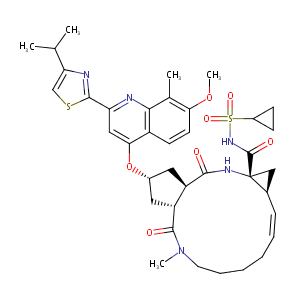
Simeprevir FDA Label
|
| 3 |
ClinicalTrials.gov (NCT02333292) Efficacy and Safety of Therapy Against HCV Based on Direct-acting Antivirals in Real-life Conditions
|
| 4 |
ClinicalTrials.gov (NCT02404805) Drug Interaction Potential Between Dolutegravir and Simeprevir in HIV/HCV Seronegative Volunteers
|
| 5 |
ClinicalTrials.gov (NCT01813513) Study to Evaluate Drug-Drug Interaction Between IDX719 and Simeprevir in Healthy Participants (MK-1894-004)
|
| 6 |
ClinicalTrials.gov (NCT01628692) Study of Daclatasvir (BMS-790052) and Simeprevir (TMC435) in Patients With Genotype 1 Chronic Hepatitis C Virus
|
| 7 |
ClinicalTrials.gov (NCT02421211) A Study to Investigate the Pharmacokinetic Interactions Between Simeprevir and Ledipasvir in a Treatment Regimen Consisting of Simeprevir, Sofosbuvir, and Ledipasvir in Treatment-naive Participants With Chronic Hepatitis C Virus Genotype 1 Infection
|
| 8 |
ClinicalTrials.gov (NCT02262728) An Efficacy, Safety and Pharmacokinetics Study of Simeprevir, Daclatasvir and Sofosbuvir in Participants With Chronic Hepatitis C Virus Genotype 1 or 4 Infection and Decompensated Liver Disease
|
| 9 |
ClinicalTrials.gov (NCT02349048) Study to Assess Efficacy, Safety, Tolerability and Pharmacokinetics of Simeprevir, Daclatasvir and Sofosbuvir in Treatment-naive Participants With Chronic Hepatitis C Virus Genotype 1 Infection
|
| 10 |
ClinicalTrials.gov (NCT02397395) An Efficacy, Safety, Tolerability and Pharmacokinetics Study of 12 Weeks Treatment With Simeprevir and Daclatasvir in Participants With Chronic Hepatitis C Virus Genotype 1b or 4 Infection and Either Severe Renal Impairment or End-stage Renal Disease on Hemodialysis.
|
| 11 |
ClinicalTrials.gov (NCT02114151) Efficacy and Safety Study of Simeprevir in Combination With Sofosbuvir in Participants With Genotype 1 Chronic Hepatitis C Virus Infection and Cirrhosis
|
|
|
|
|
|
|