Details of the Drug Combinations
General Information of This Drug (ID: DMMCYZ8)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bimiralisib; PQR309; 1225037-39-7; PI3K-IN-2; PQR-309; UNII-6Z3QHB00LB; 6Z3QHB00LB; 5-(4,6-dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine; 5-[bis(morpholin-4-yl)-1,3,5-triazin-2-yl]-4-(trifluoromethyl)pyridin-2-amine; 5-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine; Bimiralisib [INN]; Bimiralisib [USAN]; PQR309; Bimiralisib; Bimiralisib [WHO-DD]; NCB5; SCHEMBL1309049; GTPL8383; PQR309; Bimiralisib free base; ADGGYDAFIHSYFI-UHFFFAOYSA-N; EX-A2018; BCP15887; PQR-309(PI3K-IN-2)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
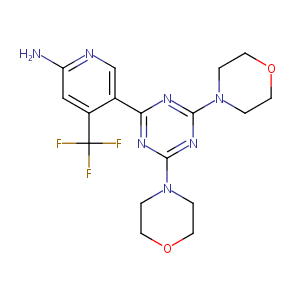 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
