Details of the Drug Combinations
General Information of This Drug (ID: DMN4OGH)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TAK-960; 1137868-52-0; TAK960; TAK 960; CHEMBL2392545; AK172385; benzamide, 4-[(9-cyclopentyl-7,7-difluoro-6,7,8,9-tetrahydro-5-methyl-6-oxo-5h-pyrimido[4,5-b][1,4]diazepin-2-yl)amino]-2-fluoro-5-methoxy-n-(1-methyl-4-piperidinyl)-; 4-[(9-Cyclopentyl-7,7-Difluoro-5-Methyl-6-Oxo-6,7,8,9-Tetrahydro-5h-Pyrimido[4,5-B][1,4]diazepin-2-Yl)amino]-2-Fluoro-5-Methoxy-N-(1-Methylpiperidin-4-Yl)benzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
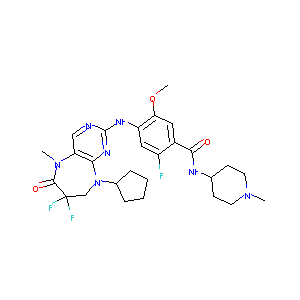 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
References
