Details of the Drug Combinations
General Information of This Drug (ID: DMO4S6T)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PF-03814735; 942490-07-5; PF 03814735; PF-3814735; PF03814735; Kinome_1769; MLS006010949; CHEMBL1983111; SB19308; NCGC00389594-01; KB-74441; SMR004703046; BCP0726000114; J3.628.238J; N-(2-(6-((4-(cyclobutylamino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-1,2,3,4-tetrahydro-1,4-epiminonaphthalen-9-yl)-2-oxoethyl)acetamide; N-[2-(4-{[4-(cyclobutylamino)-5-(trifluoromethyl)pyrimidin-2-yl]amino}-11-azatricyclo[6.2.1.0^{2,7}]undeca-2(7),3,5-trien-11-yl)-2-oxoethyl]acetamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
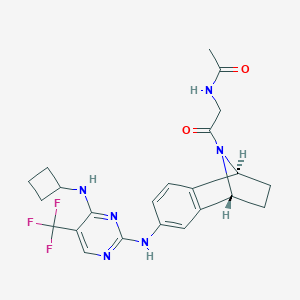 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||
References
