Details of the Drug Combinations
General Information of This Drug (ID: DMOJV2N)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tipiracil / Trifluridine; Viroptic mixture with 5-CIMU; TAS 102; EX-A1755; Tipiracil hydrochloride / Trifluridine; Tipiracil hydrochloride mixture with Trifluridine; 1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-(trifluoromethyl)pyrimidine-2,4(1H,3H)-dione compound with 5-chloro-6-((2-iminopyrrolidin-1-yl)methyl)pyrimidine-2,4(1H,3H)-dione (1:1) hydrochloride; Thymidine, alpha,alpha,alpha-trifluoro-, mixt. with 5-chloro-6-((2-imino-1-pyrrolidinyl)methyl)-2,4(1H,3H)-pyrimidinedione monohydro
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
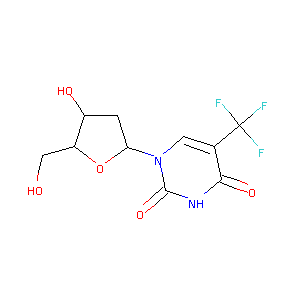 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
