Details of the Drug Combinations
General Information of This Drug (ID: DMOSW35)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Agon; Felobeta; Felocor; Feloday; Felodipina; Felodipinum; Felodur; Felogamma; Felogard; Fensel; Flodil; Hydac; Lexxel; Logimax; Modip; Munobal; Penedil; Perfudal; Plandil; Plendil; Preslow; Prevex; Renedil; Splendil; AGON SR; AbZ Brand of Felodipine; Aliud Brand of Felodipine; Alphapharm Brand of Felodipine; Alpharma Brand of Felodipine; Astra Brand of Felodipine; AstraZeneca Brand of Felodipine; Aventis Brand of Felodipine; Azupharma Brand of Felodipine; BC Brand of Felodipine; Betapharm Brand of Felodipine; Ct Arzneimittel Brand of Felodipine; Felo Biochemie; Felo Puren; Felodipin AL; Felodipin AZU; Felodipin AbZ; Felodipin Heumann; Felodipin Stada; Felodipin dura; Felodipin ratiopharm; Felodipin von ct; FelodurER; Heumann Brand of Felodipine; Hexal Brand of Felodipine; Hoechst Brand of Felodipine; Merck dura Brand of Felodipine; Munobal Retard; Pharmaceutica Astra Brand of Felodipine; Pharmacia Spain Brandof Felodipine; Plendil Depottab; Plendil ER; Plendil Retard; Promed Brand of Felodipine; Ratiopharm Brand of Felodipine; Stadapharm Brand of Felodipine; TheraPharm Brand of Felodipine; Worwag Brand of Felodipine; F 9677; Felodipin 1A Pharma; H 154 82; H 15482; CGH-869; Ct-Arzneimittel Brand of Felodipine; Dl-Felodipine; Felo-Puren; Felodipin-ratiopharm; Felodipina [INN-Spanish]; Felodipinum [INN-Latin]; H 154-82; H 154/82; Heumann, Felodipin; Plendil (TN); AE-641/11429675; Felodipine [USAN:BAN:INN]; Felodipine [USAN:INN:BAN]; H-154/82; Felodipine (JAN/USP/INN); Plendil, Renedil, Feloday, Felodipine; Ethyl methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; (+-)-Ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate; (+/-)-ethylmethyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate; 1A Brand of Felodipine; 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-4-(2,3-dichlorophenyl)-2,6-dimethyl-, ethyl methylester; 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-4-(2,3-dichlorophenyl)-2,6-dimethyl-,ethyl methyl ester; 3,5-Pyridinedicarboxylic acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, ethyl methyl ester; 3-Ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate; 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; 4-(2,3-Dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinecarboxylic acid ethyl methyl ester; 4-(2,3-dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid 3-ethyl ester 5-methyl ester; 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid ethyl methyl ester; 5-O-ethyl 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
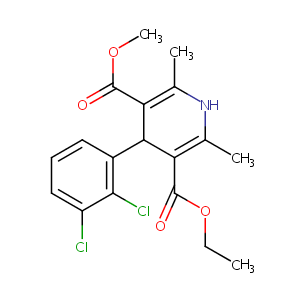 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
