Details of the Drug Combinations
General Information of This Drug (ID: DMOTQ1I)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DESMETHYLONDANSETRON; Zofran; Zophren; Zudan; Sandoz ondansetron; ZOFRAN IN PLASTIC CONTAINER; Zofran ODT; GR 38032; GR 38032X; GR38032F; Apo-ondansetron; GR-38032F; Novo-ondansetron; Ondansetron (Zofran); PHL-ondansetron; PMS-ondansetron; Ratio-ondansetron; SN-307; Zofran (TN); Zofran ODT (TN); Ondansetron [USAN:INN:BAN]; Ondansetron (JAN/USP/INN); Ondansetron, (+,-)-Isomer; (RS)-1,2,3,9-Tetrahydro-9-methyl-3-(2-methylimidazol-1-ylmethyl)carbazol-4-one; 1,2,3,9-Tetrahydro-9-methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-4H-carbazol-4-one; 9-Methyl-3-(2-methyl-imidazol-1-ylmethyl)-1,2,3,9-tetrahydro-carbazol-4-one; 9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one; 9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1H-carbazol-4-one
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiemetics
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
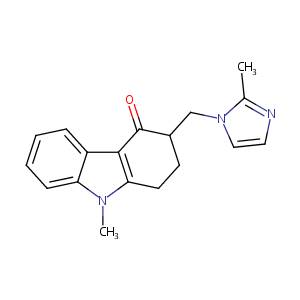 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
24 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
