Details of the Drug Combinations
General Information of This Drug (ID: DMQ4HIN)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cabaser; Cabaseril; Cabergolina; Cabergolinum; Dostinex; Galastop; Sogilen; Cabergolina [Spanish]; Cabergolinum [Latin]; CG-101; Cabaser (TN); Dostinex (TN); FCE-21336; Cabergoline [USAN:BAN:INN]; Cabergoline (JAN/USAN/INN); (8R)-6-allyl-N-[3-(dimethylamino)propyl]-N-(ethylcarbamoyl)ergoline-8-carboxamide; (8beta)-N-[3-(dimethylamino)propyl]-N-(ethylcarbamoyl)-6-(prop-2-en-1-yl)ergoline-8-carboxamide; (8beta)-N-[3-(dimethylamino)propyl]-N-[(ethylamino)carbonyl]-6-(2-propenyl)-ergoline-8-carboxamide; (8beta)-N-[3-(dimethylamino)propyl]-N-[(ethylamino)carbonyl]-6-prop-2-en-1-ylergoline-8-carboxamide; 1-((6-Allylergolin-8beta-yl)carbonyl)-1-(3-(dimethylamino)propyl)-3-ethylurea; 1-[(6-allylergoline-8beta-yl)carbonyl]-1-[3-(dimethylamino)propyl]-3-ethylurea; 1-ethyl-2-(3'-dimethylaminopropyl)-3-(6'-allylergoline-8'-beta-carbonyl)urea diphosphate; 1-ethyl-3-(3'-dimethylamionpropyl)-2-(6'-allylergoline-8'beta-carbonyl)urea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
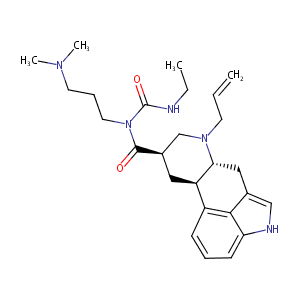 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
