Details of the Drug Combinations
General Information of This Drug (ID: DMQ8J0U)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1159600-41-5; UNII-788PET5SUA; 788PET5SUA; (1,1-Dioxidothiomorpholino)(6-((3-(4-fluorophenyl)-5-methylisoxazol-4-yl)methoxy)pyridin-3-yl)methanone; Basmisanil [INN]; Basmisanil [USAN:INN]; Basmisani; Basmisanil(RG1662); Basmisanil (USAN/INN); SCHEMBL2685527; CHEMBL3681419; MolPort-044-561-818; VCGRFBXVSFAGGA-UHFFFAOYSA-N; BDBM133427; EX-A1272; AKOS032947142; ZINC145814743; DB11877; CS-6046; HY-16716; (1,1-Dioxo-4-thiomorpholinyl)(6-((3-(4-fluorophenyl)-5-methylisoxazol-4-yl)methoxy)pyridin-3-yl)metha
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Structure |
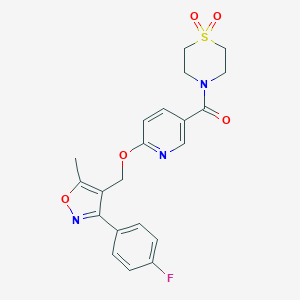 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
References
