Details of the Drug Combinations
General Information of This Drug (ID: DMQM2FH)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
RRX-001; 925206-65-1; 2-BROMO-1-(3,3-DINITROAZETIDIN-1-YL)ETHAN-1-ONE; UNII-7RPW6SU9SC; 2-Bromo-1-(3,3-dinitroazetidin-1-yl)ethanone; 7RPW6SU9SC; RRx001; Ethanone, 2-bromo-1-(3,3-dinitro-1-azetidinyl)-; SCHEMBL2249018; CHEMBL3526802; MolPort-044-559-098; EX-A2006; BCP19228; 1-bromoacetyl-3,3-dinitroazetidine; ZINC34805177; s8405; DB12060; CS-5286; HY-16438; AS-53015
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
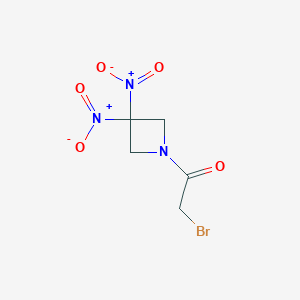 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
