Details of the Drug Combinations
General Information of This Drug (ID: DMR6LXM)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TT-232; UNII-49D4Q4254Z; TT 232; 49D4Q4254Z; 147159-51-1; Phe-cys-tyr-trp-lys-cys-thr-NH2 (2-6)-disulfide; TLN 232; CAP-232; CAP 232; Phenylalanyl-cysteinyl-tyrosyl-tryptophyl-lysyl-cysteinyl-threoninamide (2-6)-disulfide; AC1OCF7X; CHEMBL539934; TLN-232; L-Threoninamide, D-phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-cysteinyl-, cyclic (2-6)-disulfide; TT2-32; ZINC169289417; AKOS024458270; DB12088; NCGC00249606-01
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
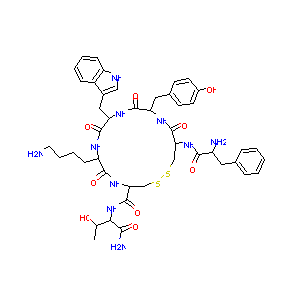 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
References
