Details of the Drug Combinations
General Information of This Drug (ID: DMRKXPT)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Actron; Alrheumat; Alrheumum; Alrheumun; Aneol; Capisten; Dexal; Epatec; Fastum; Kefenid; Ketoprofene; Ketoprofeno; Ketoprofenum; Ketopron; Ketoprophene; Lertus; Menamin; Meprofen; Orudis; Orugesic; Oruvail; Oscorel; Profenid; Toprec; Toprek; Benzoylhydratropic Acid; Orudis KT; K 1751; RP 19583; RP19583; RU 4733; Arthril (TN); Fastum (TN); Fastum Gel (TN); Iso-K; Keto (TN); Ketoflam (TN); Ketomex (TN); Ketonal (TN); Ketoprofenas (TN); Ketoprofene (TN); Ketoprofene [INN-French]; Ketoprofeno [INN-Spanish]; Ketoprofenum (TN); Ketoprofenum [INN-Latin]; Ketorin (TN); Ketospray (TN); Lasonil (TN); M-Benzoylhydratropic acid; Oki (TN); Orudis (TN); Oruvail (TN); RP-19583; Racemic-Ketoprofen; Zon (TN); Bi-Profnid (TN); RP, 19,583; Ketoprofen (JP15/USP/INN); Ketoprofen [USAN:INN:BAN:JAN]; Orudis, Oruvail, Ketoflam, Orudis KT, Ketoprofen; Acide (benzoyl-3-phenyl)-2-propionique; Acide (benzoyl-3-phenyl)-2-propionique [French]; L'Acide (benzoyl-3-phenyl)-2-propionique; (+-)-m-Benzoylhydratropic acid; 2-(3-Benzoylphenyl)propanoic acid; 2-(3-Benzoylphenyl)propionic acid; 2-(m-Benzoylphenyl)propionic acid; 2-[3-(benzoyl)phenyl]propanoic acid; 2-[3-(phenylcarbonyl)phenyl]propanoic acid; 2-[3-Benzoylphenyl]propionic acid; 3-Benzoylhydratropic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
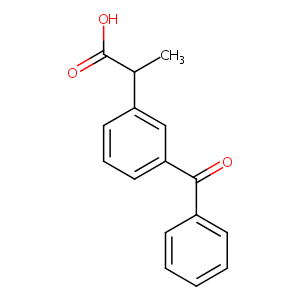 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
