Details of the Drug Combinations
General Information of This Drug (ID: DMSFK9V)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1009119-64-5; Daclatasvir (BMS-790052); 1214735-16-6; CHEBI:82977; C40H50N8O6; methyl N-[(1S)-1-[(2S)-2-[5-[4-[4-[2-[(2S)-1-[(2S)-2-(methoxycarbonylamino)-3-methyl-butanoyl]pyrrolidin-2-yl]-1H-imidazol-5-yl]phenyl]phenyl]-1H-imidazol-2-yl]pyrrolidine-1-carbonyl]-2-methyl-propyl]carbamate; Daclatasvir (USAN); cc-39; Daclatasvir BMS 790052; MLS006011140; SCHEMBL2756027; CHEMBL2023898; SCHEMBL17897804; KS-00000XPC; EX-A410
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
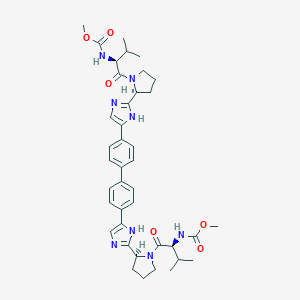 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
11 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
