Details of the Drug Combinations
General Information of This Drug (ID: DMSH4DG)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
silibinin; Silybin; 22888-70-6; Flavobin; Silymarin I; Silybin A; Silybine; Silibinine; Silliver; Silibinin A; Silibininum; Silibinina; Flavobin Spofa; Silymarine I; Silibinin [INN]; Silymarine; Silibininum [INN-Latin]; Silibinine [INN-French]; Silibinina [INN-Spanish]; 7C3MT; UNII-33X338MNE4; EINECS 245-302-5; NSC 651520; Silimarin; Silibin; CHEBI:9144; 33X338MNE4; Silibinin (INN); NSC651520; C25H22O10; NCGC00091057-01; DSSTox_CID_6018; DSSTox_RID_77985; DSSTox_GSID_26018; (2R,3R)-3,5,7-trihydroxy-2-((2R,3R)-3-(4-hydroxy-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
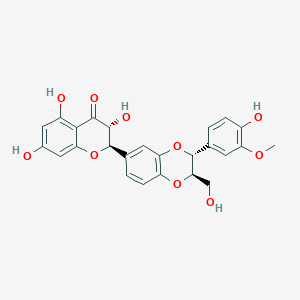 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
