Details of the Drug Combinations
General Information of This Drug (ID: DMT6E5N)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Onapristone; 96346-61-1; Onapristone [INN]; ZK-98299; Onapristonum [Latin]; Onapristona [Spanish]; UNII-H6H7G23O3N; ZK 299; CCRIS 6530; ZK 98299; ZK-299; H6H7G23O3N; C29H39NO3; Onapristone (INN); Onapristonum; Onapristona; (8S,11R,13R,14S,17S)-11-(4-dimethylaminophenyl)-17-hydroxy-17-(3-hydroxypropyl)-13-methyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one; Estra-4,9-dien-3-one, 11-(4-(dimethylamino)phenyl)-17-hydroxy-17-(3-hydroxypropyl)-, (11-beta,13-alpha,17-alpha)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
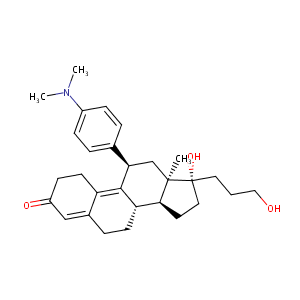 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
