| 1 |
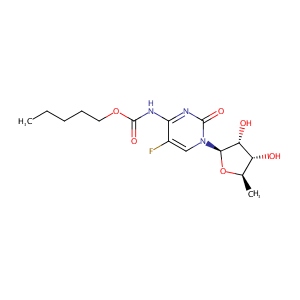
Capecitabine FDA Label
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6799).
|
| 3 |
ClinicalTrials.gov (NCT01589419) A Clinical Study Conducted in Multiple Centers Evaluating Escalating Doses of Veliparib in Combination With Capecitabine and Radiation in Patients With Locally Advanced Rectal Cancer
|
| 4 |
ClinicalTrials.gov (NCT01843725) Phase 1 Study Testing the Combination of Aflibercept and Capecitabine in Metastatic Digestive and Breast Cancers
|
| 5 |
ClinicalTrials.gov (NCT01300962) Study of BKM120 or BYL719 and Capecitabine in Patients With Metastatic Breast Cancer
|
| 6 |
ClinicalTrials.gov (NCT02550743) BrUOG 302:BYL719, Capecitabine and Radiation for Rectal Cancer: A Brown University Oncology Research Group Study
|
| 7 |
ClinicalTrials.gov (NCT04664829) The Role of Bexarotene in Inducing Susceptibility to Chemotherapy in Metastatic TNBC
|
| 8 |
ClinicalTrials.gov (NCT00959946) Study Of Bosutinib With Capecitabine In Solid Tumors And Locally Advanced Or Metastatic Breast Cancer
|
| 9 |
ClinicalTrials.gov (NCT00121277) Vorinostat and Capecitabine in Treating Patients With Metastatic or Unresectable Solid Tumors
|
| 10 |
ClinicalTrials.gov (NCT01494155) Short Course Radiation Therapy With Proton or Photon Beam Capecitabine and Hydroxychloroquine for Resectable Pancreatic Cancer
|
| 11 |
ClinicalTrials.gov (NCT00130507) Benefit of Adding Trastuzumab to Second Line Chemotherapy in Breast Cancer Patients Previously Treated With Trastuzumab
|
| 12 |
ClinicalTrials.gov (NCT00398879) Placebo-Controlled Study of Perifosine + Single Agent Chemotherapy for Metastatic Cancer Patients
|
| 13 |
ClinicalTrials.gov (NCT00661102) A Study of Prophylactic Treatment for Hand-Foot Syndrome in Patients Treated With Oral Xeloda (Capecitabine).
|
| 14 |
ClinicalTrials.gov (NCT00452673) Phase I Study of Dasatinib (BMS-354825) and Capecitabine for Women With Advanced Breast Cancer
|
| 15 |
ClinicalTrials.gov (NCT01267240) Capecitabine and Vorinostat in Treating Patients With Recurrent and/or Metastatic Head and Neck Cancer
|
| 16 |
ClinicalTrials.gov (NCT02352831) Tosedostat With Capecitabine in Patients With Metastatic Pancreatic Adenocarcinoma
|
| 17 |
ClinicalTrials.gov (NCT02422199) A Study of Pyrotinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in Patients With HER2+Metastatic Breast Cancer Who Have Prior Received Anthracyclin, Taxane or Trastuzumab
|
| 18 |
ClinicalTrials.gov (NCT02861300) CB-839 + Capecitabine in Solid Tumors and Fluoropyrimidine Resistant PIK3CA Mutant Colorectal Cancer
|
| 19 |
ClinicalTrials.gov (NCT00305643) Celecoxib in Preventing Hand/Foot Syndrome Caused By Capecitabine With Metastatic Breast or Colorectal Cancer
|
| 20 |
ClinicalTrials.gov (NCT00081224) Neoadjuvant Celecoxib and Capecitabine Combined With Pelvic Irradiation in Treating Patients With Stage II or Stage III Adenocarcinoma (Cancer) of the Rectum
|
| 21 |
ClinicalTrials.gov (NCT00107276) S0430 Cyclophosphamide and Capecitabine in Treating Women With Stage IV Breast Cancer
|
| 22 |
ClinicalTrials.gov (NCT00050167) Evaluation of Differing Taxanes/Taxane Combinations on the Outcome of Patients With Operable Breast Cancer
|
| 23 |
ClinicalTrials.gov (NCT00054457) Docetaxel and Capecitabine in Treating Patients With Metastatic Cancer of the Stomach or Gastroesophageal Junction
|
| 24 |
ClinicalTrials.gov (NCT00437294) Enzastaurin in Combination of Capecitabine to Treat Breast Cancer
|
| 25 |
ClinicalTrials.gov (NCT01323530) A Confirmation Study of Eribulin in Combination With Capecitabine
|
| 26 |
ClinicalTrials.gov (NCT00440167) Capecitabine/Erlotinib Followed of Gemcitabine Versus Gemcitabine/Erlotinib Followed of Capecitabine
|
| 27 |
ClinicalTrials.gov (NCT01099527) A Trial of RAD001/Capecitabine in Refractory Gastric Cancer
|
| 28 |
ClinicalTrials.gov (NCT00037609) Safety, Efficacy and Pharmacokinetic Between Capecitabine and Exisulind in Metastatic Breast Cancer Patients
|
| 29 |
ClinicalTrials.gov (NCT00534417) Phase II Trial of Capecitabine With Fulvestrant for Postmenopausal Women With Hormone Receptor Positive Metastatic Breast Cancer
|
| 30 |
ClinicalTrials.gov (NCT01332604) GDC-0980 in Combination With a Fluoropyrimidine, Oxaliplatin, and Bevacizumab in Patients With Advanced Solid Tumors
|
| 31 |
ClinicalTrials.gov (NCT00039390) Gefitinib and Capecitabine in Treating Patients With Advanced Solid Tumors
|
| 32 |
ClinicalTrials.gov (NCT00626158) Gemcitabine and Capecitabine to Treat Patients With Advanced Pancreatic and Biliary Cancers
|
| 33 |
ClinicalTrials.gov (NCT00741260) Study Evaluating The Combination Of Neratinib And Capecitabine In Solid Tumors And Breast Cancer
|
| 34 |
ClinicalTrials.gov (NCT00087152) S0338, Imatinib Mesylate and Capecitabine in Treating Women With Progressive Stage IV Breast Cancer
|
| 35 |
ClinicalTrials.gov (NCT00726687) Phase 1b Study of Indibulin in Combination With Capecitabine in Advanced Solid Tumors
|
| 36 |
ClinicalTrials.gov (NCT00022698) Capecitabine and Irinotecan in Treating Patients With Locally Advanced, Recurrent, or Metastatic Colorectal Cancer
|
| 37 |
ClinicalTrials.gov (NCT00568022) A Phase I Study of Ixabepilone in Combination With Capecitabine in Japanese Patients With Metastatic Breast Cancer
|
| 38 |
ClinicalTrials.gov (NCT01349088) Investigational Drug in Combination With Two Chemotherapy Drugs in Women With Locally Recurrent or Metastatic Breast Cancer
|
| 39 |
ClinicalTrials.gov (NCT00078572) Capecitabine (XELODA) With Or Without Lapatinib (GW572016) For Women With Refractory Advanced or Metastatic Breast Cancer
|
| 40 |
ClinicalTrials.gov (NCT00327743) Combination Study of a New Taxane and Capecitabine in Patients With Metastatic Breast Cancer
|
| 41 |
ClinicalTrials.gov (NCT02915172) Lenvatinib and Capecitabine in Patients With Advanced Malignancies
|
| 42 |
ClinicalTrials.gov (NCT02210364) Study of Lurbinectedin (PM01183) in Combination With Capecitabine in Patients With Metastatic Breast Cancer (MBC), Pancreatic Cancer (PC) or Metastatic Colorectal Cancer (CRC).
|
| 43 |
ClinicalTrials.gov (NCT06156761) Mitoxantrone Hydrochloride Liposome Combined With Capecitabine in Patients With HER-2 Negative Advanced Breast Cancer
|
| 44 |
ClinicalTrials.gov (NCT02244489) Momelotinib Combined With Capecitabine and Oxaliplatin in Adults With Relapsed/Refractory Metastatic Pancreatic Ductal Adenocarcinoma
|
| 45 |
ClinicalTrials.gov (NCT01776307) A Study of BBI608 in Adult Patients With Advanced Colorectal Cancer
|
| 46 |
ClinicalTrials.gov (NCT00040859) Oxaliplatin and Capecitabine in Treating Patients With Advanced Esophageal Cancer or Stomach Cancer
|
| 47 |
ClinicalTrials.gov (NCT00058474) Radiation Therapy and Either Capecitabine or Fluorouracil With or Without Oxaliplatin Before Surgery in Treating Patients With Resectable Rectal Cancer
|
| 48 |
ClinicalTrials.gov (NCT00632489) LBH589 in Combination With Capecitabine Plus/Minus () Lapatinib in Breast Cancer Patients
|
| 49 |
ClinicalTrials.gov (NCT02910843) Neoadjuvant Treatment With Regorafenib and Capecitabine Combined With Radiotherapy in Locally Advanced Rectal Cancer
|
| 50 |
ClinicalTrials.gov (NCT01423604) Study of Ruxolitinib in Pancreatic Cancer Patients
|
| 51 |
ClinicalTrials.gov (NCT01134601) A Phase I Study of AZD6244 in Combination With Capecitabine and Radiotherapy in Locally Advanced Adenocarcinoma of the Rectum
|
| 52 |
ClinicalTrials.gov (NCT03473639) A Pilot Study of the Combination of Entinostat With Capecitabine in High Risk Breast Cancer After Neo-adjuvant Therapy
|
| 53 |
ClinicalTrials.gov (NCT00662025) Study Of Sunitinib With Capecitabine In Breast Cancer
|
| 54 |
ClinicalTrials.gov (NCT00570908) Brain Mets - Capecitabine Plus Sunitinib and WBRT
|
| 55 |
ClinicalTrials.gov (NCT00869050) Capecitabine and Temozolomide for Neuroendocrine Cancers
|
| 56 |
ClinicalTrials.gov (NCT03213002) Oral Capecitabine and Temozolomide (CAPTEM) for Newly Diagnosed GBM
|
| 57 |
ClinicalTrials.gov (NCT01573468) Capecitabine/Tesetaxel Versus Capecitabine/Placebo as Second-line Therapy for Gastric Cancer
|
| 58 |
ClinicalTrials.gov (NCT02025192) A Study of Tucatinib (ONT-380) Combined With Capecitabine and/or Trastuzumab in Patients With HER2+ Metastatic Breast Cancer
|
| 59 |
ClinicalTrials.gov (NCT01898104) Preoperative Valproic Acid and Radiation Therapy for Rectal Cancer
|
| 60 |
ClinicalTrials.gov (NCT03082053) A Study of Varlitinib in Japanese Subjects With Advanced or Metastatic Solid Tumours
|
| 61 |
ClinicalTrials.gov (NCT04274933) A Study to Evaluate the Safety and Tolerability of Venetoclax Tablets in Combination With Capecitabine Tablets in Adult Participants With Hormone Receptor-Positive, HER2-Negative Locally Advanced or Metastatic Breast Cancer Who Had Disease Progression During or After CDK4/6 Inhibitor Therapy
|
| 62 |
ClinicalTrials.gov (NCT00450515) Vinflunine and Capecitabine in Treating Patients With Previously Treated Metastatic Breast Cancer
|
| 63 |
ClinicalTrials.gov (NCT00077363) Capecitabine and Tipifarnib in Treating Women With Taxane-Resistant Metastatic Breast Cancer
|
| 64 |
ClinicalTrials.gov (NCT05089643) Anrotinib in Combination With Capecitabine in Advanced Triple Negative Breast Cancer
|
| 65 |
ClinicalTrials.gov (NCT04464174) Ipatasertib Plus Non-Taxane Chemotherapy for Advanced or Metastatic Triple-Negative Breast Cancer
|
| 66 |
ClinicalTrials.gov (NCT01993472) Study of Andrographolides With or Without Capecitabine to Treat Colorectal Cancer
|
| 67 |
ClinicalTrials.gov (NCT05062317) ctDNA-Directed Post-Hepatectomy Chemotherapy for Patients With Resectable Colorectal Liver Metastases
|
| 68 |
ClinicalTrials.gov (NCT01887288) Capecitabine With Digoxin for Metastatic Breast Cancer
|
| 69 |
ClinicalTrials.gov (NCT05833919) Second Line ERIbulin Followed by CApecitabine or the Reverse Sequence in HER2-negative Metastatic Breast Cancer Patients
|
| 70 |
ClinicalTrials.gov (NCT02767752) Gemcitabine and Capecitabine With or Without T-ChOS as Adjuvant Therapy for Patients With Resected Pancreatic Cancer
|
| 71 |
ClinicalTrials.gov (NCT00216086) Capecitabine + Irinotecan Followed by Combined Modality Capecitabine and Radiation in Locally Advanced Rectal Cancer
|
| 72 |
ClinicalTrials.gov (NCT01843452) Phase II Study of Concomitant Intensity-modulated Radiotherapy Combined to Capecitabine, Mitomycin and Panitumumab in Patients With Stage II-IIIB Squamous-cell Carcinoma of the Anal Canal
|
| 73 |
ClinicalTrials.gov (NCT02780700) Nintedanib Alone or in Combination With Capecitabine in Refractory Metastatic Colorectal Cancer [LUME-Colon 2]
|
| 74 |
ClinicalTrials.gov (NCT03817411) Telatinib in Combination With Capecitabine/ Oxaliplatin in 1st Line Gastric or GEJ Cancer
|
| 75 |
ClinicalTrials.gov (NCT02474186) Phase II Study for Solid Metastatic Tumors
|
| 76 |
ClinicalTrials.gov (NCT04034251) Intraperitoneal and Intravenous Paclitaxel Chemotherapy With Oral Capecitabine for Gastric Adenocarcinoma With Peritoneal Carcinomatosis
|
| 77 |
ClinicalTrials.gov (NCT02955940) An Open-Label Study to Enable Continued Treatment Access for Subjects Previously Enrolled in Studies of Ruxolitinib
|
| 78 |
ClinicalTrials.gov (NCT03114085) Apatinib Combined With Capecitabine Compared With Apatinib Treat Advanced Hepatocellular Carcinoma
|
| 79 |
ClinicalTrials.gov (NCT03609489) A Study to Investigate Apatinib Combined Capecitabine on Adjuvant Therapy of Biliary Carcinoma
|
| 80 |
ClinicalTrials.gov (NCT03858972) Tesetaxel Plus Reduced Dose of Capecitabine in Patients With HER2 Negative, HR Positive, LA/MBC
|
| 81 |
ClinicalTrials.gov (NCT04721977) A Study of Tucatinib (MK-7119) in Combination With Trastuzumab and Capecitabine in Participants With Previously Treated Locally Advanced Unresectable or Metastatic Human Epidermal Growth Factor Receptor 2 Positive (HER2+) Breast Carcinoma (MK-7119-001)
|
| 82 |
ClinicalTrials.gov (NCT03093870) Varlitinib in Combination With Capecitabine for Advanced or Metastatic Biliary Tract Cancer
|
| 83 |
ClinicalTrials.gov (NCT02973737) A Study of Pyrotinib Plus Capecitabine in Patients With HER2+ Metastatic Breast Cancer
|
| 84 |
ClinicalTrials.gov (NCT05063786) Trastuzumab + Alpelisib +/- Fulvestrant vs Trastuzumab + CT in Patients With PIK3CA Mutated Previously Treated HER2+ Advanced BrEasT Cancer (ALPHABET)
|
| 85 |
ClinicalTrials.gov (NCT02767661) Metronomic Capecitabine Plus Aromatase Inhibitor for First Line Treatment in HR(+), Her2(-) Metastatic Breast Cancer
|
| 86 |
ClinicalTrials.gov (NCT04103697) Neoadjuvant Chemotherapy in Patients With Intermediate Risk Upper and Mid Rectal Cancer
|
| 87 |
ClinicalTrials.gov (NCT02119663) A Study of Ruxolitinib in Pancreatic Cancer Patients
|
|
|
|
|
|
|