Details of the Drug Combinations
General Information of This Drug (ID: DMUBP9O)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Basen; Glustat; Vocarb; Basen OD; A-71100; AO-128; Basen (TN); Basen (Takeda Chemical Industries); Volix (Ranbaxy labs);Voglibose (JP15/USAN/INN); N-(1,3-Dihydroxyprop-2-yl)valiolamine; (1S,2S,3R,4S,5S)-5-(1,3-dihydroxypropan-2-ylamino)-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol; (1S,2S,3R,4S,5S)-5-{[2-hydroxy-1-(hydroxymethyl)ethyl]amino}-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol; 3,4-Dideoxy-4-{[2-hydroxy-1-(hydroxymethyl)ethyl]amino]-2-D-epi-inositol
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Hypoglycemic Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
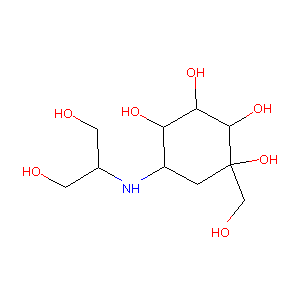 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
