Details of the Drug Combinations
General Information of This Drug (ID: DMUL5EW)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
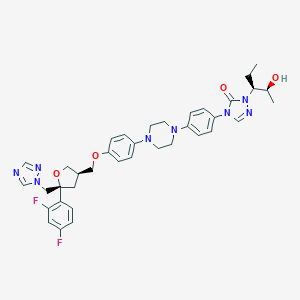
Noxafil; Spriafil; Posaconazole SP; Posaconazole in combination with MGCD290; SCH56592; Sch 56592; X2N; Noxafil (TN); Noxafil, Posaconazole; SCH-56592; Posaconazole (USAN/INN); Posaconazole [USAN:INN:BAN]; 1-((1S,2S)-1-Ethyl-2-hydroxypropyl)-4-{4-[4-(4-{[(5S,3R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazolylmethyl)oxolan-3-yl]methoxy}phenyl)piperazinyl]phenyl}-1,2,4-triazolin-5-one; 4-(p-(4-(p-(((3R,5R)-5-(2,4-Difluorophenyl)tetrahydro-5-(1H-1,2,4-triazol-1-ylmethyl)-3-furyl)methoxy)phenyl)-1-piperazinyl)phenyl)-1-((1S,2S)-1-ethyl-2-hydroxypropyl)-delta(sup 2)-1,2,4-triazolin-5-one; 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one; 4-[4-[4-[4-[[(5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
