Details of the Drug Combinations
General Information of This Drug (ID: DMVLXMG)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Camostat (mesylate); Camostat Methanesulfonate; Camostat methanesulfate; DSSTox_CID_238; DSSTox_GSID_20238; DSSTox_RID_75452; FOY 305; FOY-305; FOY305; Foipan; Foipan (TN); Foipan mesylate; NCGC00167526-01; Q-200778; UNII-451M50A1EQ; camostat mesilate; 451M50A1EQ; C21H26N4O8S; CAS-59721-29-8; CHEMBL85164
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
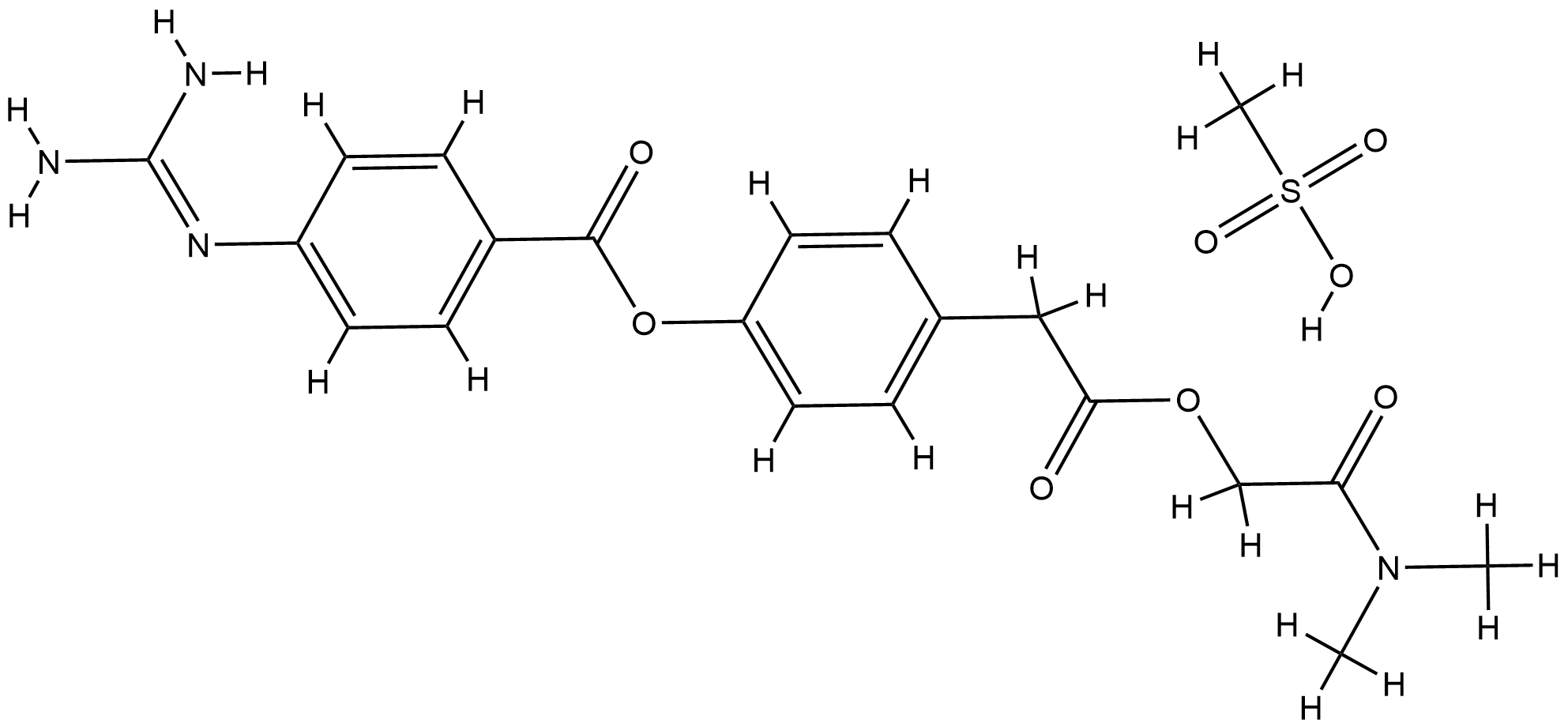 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
