Details of the Drug Combinations
General Information of This Drug (ID: DMXT7WG)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
522-17-8; (-)-Deguelin; (-)-cis-deguelin; DEGUELIN(-); UNII-K5Z93K66IE; CHEBI:4357; K5Z93K66IE; MFCD01740600; C23H22O6; (7as,13as)-13,13a-dihydro-9,10-dimethoxy-3,3-dimethyl-3h-bis[1]benzopyrano[3,4-b:6',5'-e]pyran-7(7ah)-one; (7aS,13aS)-9,10-Dimethoxy-3,3-dimethyl-13,13a-dihydro-3H-pyrano[2,3-c:6,5-f']dichromen-7(7aH)-one; (7aS,13aS)-9,10-dimethoxy-3,3-dimethyl-13,13a-dihydro-3H-pyrano[2,3-c:6,5-f']dichromen-7(7aH)-one.; SR-01000597503; CCRIS 8104; Deguelin/; (-)-Deguelin, Mundulea sericea; Spectrum_001044; Tocris-1770; Spectrum2_000298; Spectrum3_001122; Spectrum4_001965; Spectrum5_001852; SCHEMBL73183; BSPBio_002583; KBioGR_002434; KBioSS_001524; SPECTRUM201138; MLS006010295; SPBio_000236; CHEMBL393417; KBio2_001524; KBio2_004092; KBio2_006660; KBio3_002083; DTXSID10200231; HMS1923A05; HMS3268E12; 3H-Bis(1)benzopyrano(3,4-b:6',5'-e)pyran-7(7aH)-one, 13,13a-dihydro-9,10-dimethoxy-3,3-dimethyl-, (7aS-cis)-; EX-A4158; ZINC3978987; 1702AH; ABP000411; BDBM50505204; CCG-39856; LMPK12060019; s8132; AKOS024456769; ACN-053693; BCP9000596; CS-1802; SDCCGMLS-0066380.P001; NCGC00025288-01; NCGC00025288-02; NCGC00025288-03; (-)-Deguelin, >98% (HPLC), powder; AS-56004; HY-13425; SMR004701363; C10417; Q5251862; SR-01000597503-1; SR-01000597503-3; SR-01000597503-4; BRD-K61401890-001-02-0; BRD-K61401890-001-03-8; BRD-K61401890-001-04-6; (1S,14S)-17,18-dimethoxy-7,7-dimethyl-2,8,21-trioxapentacyclo[12.8.0.03,12.04,9.015,20]docosa-3(12),4(9),5,10,15,17,19-heptaen-13-one; (7aS,13aS)-9,10-Dimethoxy-3,3-dimethyl-13,13a-dihydro-3H,7aH-pyrano[2,3-c;6,5-f']dichromen-7-one; 13,13aS-Dihydro-9,10-dimethoxy-3,3-dimethyl-3H-[1]benzopyrano[3,4-b]pyrano[2,3-h][1]benzopyran-7(7aS)-one; 3H-[1]Benzopyrano[3,4-b]pyrano[2,3-h][1]benzopyran-7(7aH)-one, 13,13a-dihydro-9,10-dimethoxy-3,3-dimethyl-, (7aS,13aS)-; 3H-Bis(1)benzopyrano(3,4-b:6',5'-e)pyran-7(7aH)-one, 13,13a-dihydro-9,10-dimethoxy-3,3-dimethyl-, (7aS,13aS)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
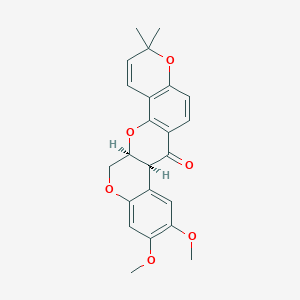 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||
References
