Details of the Drug Combinations
General Information of This Drug (ID: DMYLBOU)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
380899-24-1; UNII-1L1V1K2M4V; SB 649868; 1L1V1K2M4V; SB649868; GTPL4461; N-[[(2S)-1-[5-(4-fluorophenyl)-2-methyl-1,3-thiazole-4-carbonyl]piperidin-2-yl]methyl]-1-benzofuran-4-carboxamide; SCHEMBL8045969; CHEMBL1272307; DTXSID90191491; EX-A2570; BDBM50417257; AKOS027323765; CS-7584; SB19110; HY-10806; SB-649,868; N-((1-((5-(4-Fluorophenyl)-2-methyl-4-thiazolyl)carbonyl)-2-piperidinyl)methyl)-4-benzofurancarboxamide; 4-Benzofurancarboxamide, N-(((2S)-1-((5-(4-fluorophenyl)-2-m
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
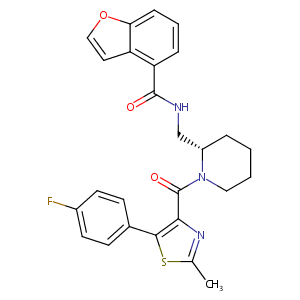 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
