Details of the Drug Combinations
General Information of This Drug (ID: DMZA5PQ)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aldiab; Digrin; Dipazide; Glibenese; Glibetin; Glican; Glide; Glidiab; Glidiazinamide; Glipid; Glipizida; Glipizidum; Glucolip; Glucotrol; Glucozide; Glupitel; Glupizide; Glyde; Glydiazinamide; Glypidizine; Melizide; Metaglip; Mindiab; Minidab; Minidiab; Minodiab; Napizide; Ozidia; Semiglynase; Sucrazide; Alphapharm Brand of Glipizide; Glibenese Brand of Glipizide; Glipizide Kenfarma Brand; Glucotrol XL; Kenfarma Brand of Glipizide; Lacer Brand of Glipizide; Lilly Brand of Glipizide; CP 28720; K 4024; K4024; PfizerBrand 1 of Glipizide; Pfizer Brand 2 of Glipizide; TK 1320; CP 28,720; CP-28720; G-117; Glipizida [INN-Spanish]; Glipizide Extended-Release Tablets; Glipizidum [INN-Latin]; Gluco-Rite; Glucotrol (TN); K-4024; KS-1068; Samarium(III) ionophore I; Glipizide (USP/INN); Glipizide [USAN:BAN:INN]; Glucotrol XL, Glucotrol, Glipizide; N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-5-methylpyrazine-2-carboxamide; N-(2-{4-[(cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-5-methylpyrazine-2-carboxamide; N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-5-methylpyrazine-2-carboxamide; N-(4-(beta-(5-Methylpyrazine-2-carboxamido)ethyl)benzenesulphonyl)-N'-cyclohexylurea; 1-Cyclohexyl-3-((p-(2-(5-methylpyrazinecarboxamido)ethyl)phenyl)sulfonyl)urea; 1-Cyclohexyl-3-{4-[2-(5-methylpyrazine-2-carboxamido)ethyl]phenylsulfonyl}urea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Hypoglycemic Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
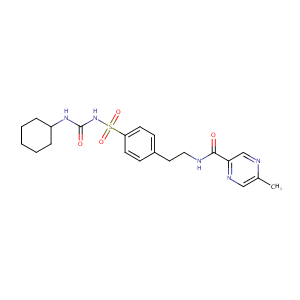 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
5 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||
References
